Mitigating unintentional effects of pharmaceutical human serum albumin preparations in therapeutic cell culture
Human Serum Albumin (HSA) has historically been used in cell culture to support mammalian cell growth. Today’s advanced therapy companies regularly depend on HSA media supplementation to help expand targeted immunotherapeutic cell lines. Albumin is known to carry many important substances including lipids, amino acids, hormones, peptides, metals, and other undefined low molecular weight molecules.1 It also offers antioxidant protection to cells and participates in the transport and signal mechanics of hormones and growth factors.1,2 It is the most abundant protein in the blood system. Clinically, HSA has been used to treat shock, acute restoration of blood volume, and hypoalbuminemia.3
There are various purified albumin products available on the market, the most prevalent being albumin derived from human blood and plasma donations.4 Human blood-derived products are extensively regulated with relevant legislation extant in every major nation and with guidance documents available from every major national and global health agency.5,6,7,8
The Use of Stabilizers in HSA
HSA has a long history of therapeutic use. Pharmaceutical HSA preparations in the United States (US) adhere to the United States Pharmacopeia (USP) monograph for human albumin, which allows for the inclusion of stabilizers sodium caprylate (a.k.a. sodium octanoate) and sodium N‐acetyl‐Tryptophanate (N-ActTryp).9 Historically, these stabilizers have been added during the manufacturing process to protect the HSA molecule while undergoing pasteurization and subsequent storage. Caprylate has the greatest stabilizing effect against heat, whereas N-ActTryp protects against the oxidation of HSA.10
While this heat treatment adds an extra layer of safety when infusing patients with therapeutic albumin preparations, it may limit the protein’s ability to stimulate and regulate the growth, differentiation, and activation of human immune cells in ex vivo applications. In ex vivo applications, one must consider cell line sensitivity to even subtle changes in a chemical environment and how this may impact drug product quality and potency.
Indeed, the same protective agents common in pharmaceutically-licensed albumin have been shown to alter the immunological response and impede cellular proliferation required for consistent cell therapy manufacturing. Caprylate has been shown to be detrimental to cell growth when present in culture media.11 The results of tryptophan degradation shown in Figure 1 (N-acetyl-kynurenine, N-formy kynurenine, and hydroxytryptophan) have been shown to be responsible for slowed and even failed cell culures.12, 13, 14
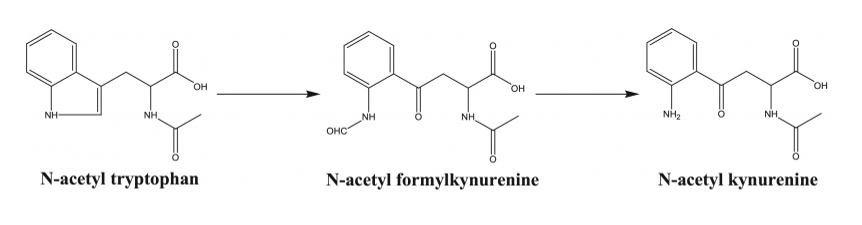
Researchers have observed inhibition and apoptosis in Natural Killer (NK92 MI) cells when treated with L-kynurenine in a dose-dependent manner.15 The kynurenine pathway has an interwoven correlation with the immune system promoting T Reg (regulatory) differentiation, which increases the production of anti-inflammatory cytokines and the suppression of the cytotoxic activity of T cells.16 It has also been reported that N-acetyl-kynurenine decreases IL-6 and CXCL-10 release from activated macrophages through the aryl hydrocarbon receptor (AhR) pathway,17 disrupting both innate and adaptive immunity.
Akron Biotech understands that end-product consistency is critical to providing a safe and efficacious therapy, allowing approval from regulatory agencies, and most importantly, providing a reliable treatment for the patient. Our AK8228 Human Serum Albumin 25% Solution is produced under Current Good Manufacturing Practices (cGMP)and all pertinent US legislation, while following global ancillary material guidance publications from relevant authoritative agencies. Akron’s manufacturing process respects and eliminates the biological influence from exogenous agents, resulting in an ideal human-derived albumin media supplement for the further manufacturing of cell and gene therapies for human treatment. Our HSA 25% Master File with the U.S. Food and Drug Administration (FDA) is available for cross-reference in your regulatory filings.
Questions?
Click here to contact our team and an Akron representative will be in touch with you shortly.
References
1. Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology. 2010;62(1):1-16. doi:10.1007/s10616-010-9263-3
2. Taverna M, Marie A-L, Mira J-P, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3(1):4. doi:10.1186/2110-5820-3-4
3. Bar-Or D, Bar-Or R, Rael LT, Gardner DK, Slone DS, Craun ML. Heterogeneity and oxidation status of commercial human albumin preparations in clinical use*: Crit Care Med. 2005;33(7):1638-1641. doi:10.1097/01.CCM.0000169876.14858.91
4. Global Albumin Market Size, Trends & Analysis – Forecasts to 2026. Global Market Estimates Research & Consultants (GME). 2020
5. U.S. Code of Federal Regulations (CFR). Title 21 part 610, General Biological Products Standards
6. Guideline on plasma-derived medicinal products. European Medicines Agency Committee for medicinal products for human use (CHMP). EMA/CHMP/BWP/706271/2010. 2011
7. Standards for Biological Materials. Pharmaceuticals and Medical Devices Agency (PMDA) Ministry of Health, Labour and Welfare (MHLW), Japan. 2014
8. World Health Organization (WHO) guidelines on Good Manufacturing Practices for Blood Establishments. Annex 4. WHO Technical Report Series, No. 961, 2011
9. United States Pharmacopoeia (USP) and National Formulary (NF) Monograph, Human Albumin. 2021
10. Anraku M, Tsurusaki Y, Watanabe H, Maruyama T, Kragh-Hansen U, Otagiri M. Stabilizing mechanisms in commercial albumin preparations: octanoate and N-acetyl-l-tryptophanate protect human serum albumin against heat and oxidative stress. Biochim Biophys Acta BBA – Proteins Proteomics. 2004;1702(1):9-17. doi:10.1016/j.bbapap.2004.07.002
11. Wong W-W, MacKenzie AD, Nelson VJ, Faed JM, Turner PR. Octanoate in Human Albumin Preparations Is Detrimental to Mesenchymal Stromal Cell Culture. Stem Cells Int. 2015;2015:1-8. doi:10.1155/2015/192576
12. Li Y, Polozova A, Gruia F, Feng J. Characterization of the Degradation Products of a Color-Changed Monoclonal Antibody: Tryptophan-Derived Chromophores. Anal Chem. 2014;86(14):6850-6857. doi:10.1021/ac404218t
13. Zang L, Frenkel R, Simeone J, Lanan M, Byers M, Lyubarskaya Y. Metabolomics Profiling of Cell Culture Media Leading to the Identification of Riboflavin Photosensitized Degradation of Tryptophan Causing Slow Growth in Cell Culture. Anal Chem. 2011;83(13):5422-5430. doi:10.1021/ac2009492
14. McElearney K, Ali A, Gilbert A, Kshirsagar R, Zang L. Tryptophan oxidation catabolite, N -formylkynurenine, in photo degraded cell culture medium results in reduced cell culture performance: N -Formylkynurenine Results in Reduced Cell Culture Performance. Biotechnol Prog. 2016;32(1):74-82. doi:10.1002/btpr.2198
15. Song H, Park H, Kim Y-S, et al. l-Kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int Immunopharmacol. 2011;11(8):932-938. doi:10.1016/j.intimp.2011.02.005
16. Ala M. The footprint of kynurenine pathway in every cancer: a new target for chemotherapy. Eur J Pharmacol. 2021;896:173921. doi:10.1016/j.ejphar.2021.173921
17. Rael LT, Bar-Or R, Banton KL, et al. The anti-inflammatory effect of LMWF5A and N-acetyl kynurenine on macrophages: Involvement of aryl hydrocarbon receptor in mechanism of action. Biochem Biophys Rep. 2018;15:61-67. doi:10.1016/j.bbrep.2018.06.006